Family : Crambidae

Texte © Dr Didier Drugmand
The box tree moth, an invasive species of moth, has been in the headlines of most scientific and horticultural media in recent years. To such an extent that classic Internet search engines refer the reader to tens of thousands of pages describing this butterfly, its ravages and the various means of control.
Originally from China, Cydalima perspectalis (Walker, 1859) has invaded almost all of Europe in about ten years; it has also recently been reported in Canada. This pest appeared in these regions, not as a result of fluctuations in its range linked in particular to the climate changes now affecting our planet, but, as we shall see later, because of human stupidity.
This moth was described by the English entomologist Francis Walker (1809-1874) in 1859. It was based on a single female, collected in northern China, in August 1854.

The box tree moth (Cydalima perspectalis) is an invasive species originating from China with a wingspan of 36-40 mm. Typically white with a brown border it can also be entirely brown, more or less iridescent, with a white discal spot on the forewing. Depending on the region, this is the case for 1 to 3 individuals out of 10 © Giuseppe Mazza
The specimen was kept in the collection of a certain Mr. Fortune. Walker classified this new species in the genus Phakellura and gave it the name perspectalis.
The original description, written in Latin and English, was published in an English journal. The holotype (the specimen on which the description is based) is now in the collections of the British Museum of Natural History in London. Below the specimen are labels bearing the words: /Phakellura perspectalis Walker, 1859, Holotype (by monotypy)/ “Type”, “N. China | 54.8.”, /”perspectalis Walk/, /BMNH/.
The genus Phakellura belongs to the family Crambidae, close to the well-known Pyralidae. It is very diverse, with approximately 15,000 species distributed throughout the world, of which about 300 belong to the European fauna. The two families are distinguished especially by the structure of the auditory organs. They contain many species whose caterpillars ravage ornamental and cultivated plants as well as stored foodstuffs.
Among the Pyralidae, we can cite in particular: the flour moth with 2 species : (Ephestia kuehniella and Pyralis farinalis), the cactus moth (Cactoblastis cactorum) as well as the rice moth (Corcyra cephalonica ) and, among the Crambidae, in addition to the box tree moth, the sugarcane moth (Diatraea saccharalis) and the corn moth (Ostrinia nubilalis).
Note that not all species are pests; some species are also used in biological control, such as Niphograpta albiguttalis, to control the development of the Water Hyacinth (Eichhornia crassipes), one of the 100 worst invasive species according to the International Union for Conservation of Nature (IUCN).
Within the Crambidae, the box tree moth has been placed, by different authors, in three genera of the subfamily Spilomeninae: (1) Palpita Hübner, 1808, (2) Diaphania Hübner, 1818 and (3) Glyphodes Guenée, 1854. As there was no relevant systematic argument to justify the placement of P. perspectalis within these taxa, a Russian entomologist, A.N. Streltzov, established in 2008 a new monotypic genus, Neoglyphodes, to host taxon perspectalis. But the saga of the box tree moth continued, as this systematic action was not relevant, as the new genus was not considered in the context of all Spilomeninae. A phylogenetic study in 2010 finally clarified the systematic imbroglio. In establishing the monophyly of the taxa, the authors placed several genera in synonymy, gave new definitions to taxonomic units and moved species. The box tree moth was thus placed in the genus Cydalima Lederer (1859).

A much less abundant colour variant has the forewing completely edged in brown. At rest a brown band seems to divide the wing obliquely © Giuseppe Mazza
Finally, it should be noted that Cydalima perspectalis still appears frequently in the scientific literature, especially in Asia, under the erroneous combinations of Glyphodes perspectalis, Diaphania perspectalis or Neoglyphodes perspectalis.
The Austrian entomologist Julius Lederer (1821-1870) created the name Cydalima in 1863, based on the Greek “cydalimos” which he translated into German in the original description by “ruhmvoll” or “glorious (se)”. The species name “perspectalis” probably derives from the verb “perspectare”, and is therefore interpreted as “well known or renowned”.
Cydalima perspectalis is known in French as Pyrale du buis, in English: “Box tree moth” or , in Italian: “Piralide del bosso”, in Spanish: “polilla del boj” or “piral del boj”, in Dutch: “buxusmot”, in German: “Buchsbaumzünsler” and in Portuguese: “Traça Do Buxo”.

Here the brown variant with its golden to purplish iridescence. The adults are not very active during the day and remain hidden within the foliage of the boxwood or even another plant. It is not uncommon to observe butterflies in the evening on walls or lighted display cases. This species is indeed strongly attracted by artificial lighting © Giuseppe Mazza
Possible confusion
The caterpillars of the box tree moth resemble those of the large white (Pieris brassicae (Linnaeus, 1758)) somewhat, but the latter do not feed at the expense of boxwood. Careful examination will quickly differentiate between the two species. As for the adult, it could be confused with the clouded border (Lomaspilis marginata (Linnaeus, 1758), which is however smaller and has a different wing coloration.
Zoogeography
The center of origin of Cydalima perspectalis is in East Asia, probably China. It should be noted that on the label of the type was written “N. China”.

The three chromatic morphs © Claude Galand
Since its description, the species has been reported from all over China, Taiwan, Japan and South Korea. Early citations from India still require confirmation.
The distribution of C. perspectalis in Asia is largely related to the different native and ornamental Buxus species. The abundance of the moth in northern China and the Russian Far East, where Buxus spp. do not occur naturally, is explained by the recent introduction of ornamental boxwood into these regions.
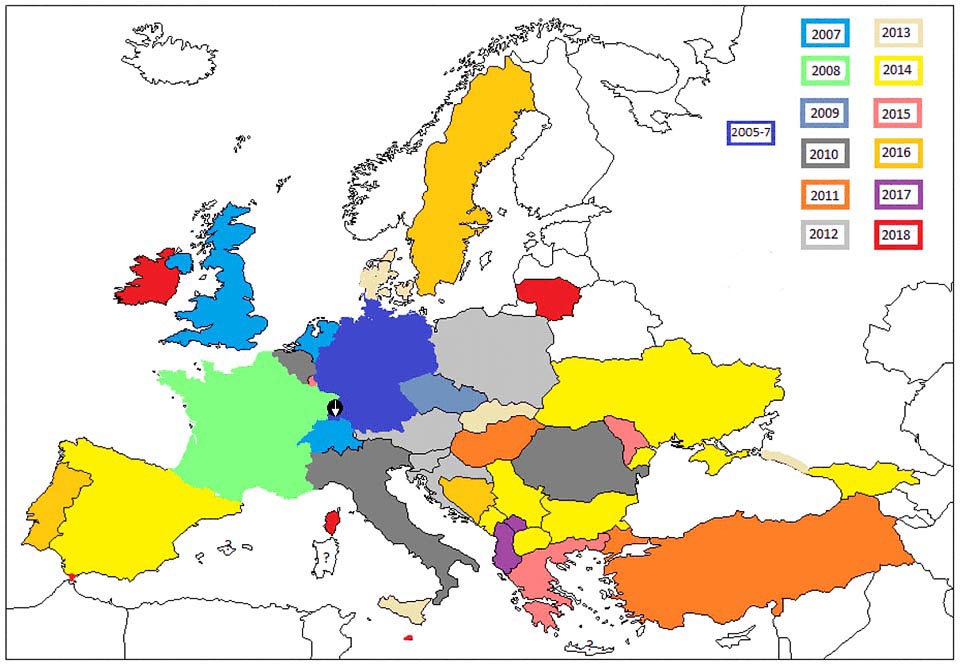
In May 2007, the first caterpillars of the box tree moth were discovered far from Asia, in south-western Germany, in and around the city of Weil am Rhein (Baden-Württemberg). These were the first validated data for continental Europe. Considering the size of the observed populations, the establishment of the codling moth was probably due to take place in 2005 (it was already reported in 2006 among nurserymen). The moth then rapidly continued its spread to new areas and is now present in more than 30 European countries.
Its spread has been favoured by the free market for live plants in the European Union and by the presence in the wild of two species of boxwood native to Europe, Buxus sempervirens and B. balearica. In the same year, observers were already pointing to C. perspectalis in Switzerland, the Netherlands and also in the United Kingdom. Let us then follow its spread: in 2008, the moth appeared in Alsace (France). In 2009, it reached the Paris region, and then covered the whole French territory by 2015. The scientific community reported it in 2009 in Austria and Lichtenstein, in 2010 in Belgium, Italy and Romania, in 2011 in Hungary and Turkey, and in 2012 in Croatia, the Czech Republic, Poland and Slovenia; in 2013 in Denmark, Sicily, Chechnya, Russia (including the Olympic city of Sochi on the shores of the Black Sea, impacted by Italy’s shipment of infected ornamental boxwood) and Slovakia, in 2014 in Ukraine, Bulgaria, Macedonia, Montenegro, Georgia, Serbia and Spain, in 2015 in Luxembourg, Moldova and Greece; in 2016 in Portugal, Bosnia and Herzegovina and southern Sweden. More recently, in 2017 in Albania and Kosovo, and in 2018 in Ireland, Corsica, Scotland, Gibraltar, Lithuania and Malta.
Cydalima perspectalis has not yet been detected in Cyprus, Estonia, Finland and Latvia. The moth can probably become established in all these countries under current climatic conditions, except for Finland, where its distribution would be limited to the warmest regions. Note that with the current global warming, the warmer areas of the boreal region could be invaded, as well as some alpine, Atlantic and continental regions that are currently still too cold for the moth to survive or complete a generation. As temperatures rise, the moth would in the future develop two generations per year in areas where it can now complete only one generation. This change will most certainly increase the damage to boxwood in these areas. To be continued!
Careful examination of the map and the chronology of observations in Europe indicates that the current distribution of the moth can only be explained by multiple introductions (linked to boxwood imports from China) and not as a result of a gradual spread (by flight of adults) from a single point of origin (south-western Germany).
Outside Europe, C. perspectalis was detected in 2018 (data officialized in 2019) in an urban area in Toronto, Ontario. The source of this introduction remains unknown, but it is likely that the moth was introduced with imported boxwood, as no Buxus is native to Canada. However, one species – Buxus vahlii Baillon, 1859 – is endemic to Puerto Rico and the U.S. Virgin Islands. Will the moth ravage this endemic boxwood in the coming years?
It has invaded almost all of Europe in about ten years. The first appearance took place in Rhein, in south-western Germany © Didier Drugmand
In 2017, the moth was reported in Pakistan. It is not yet known whether this area is in its original range or whether the moth has colonized this country secondarily.
Ecology and Habitat
In its native range, the caterpillars of C. perspectalis cause varying degrees of damage to different species of boxwood (genus Buxus family Buxaceae), mainly by consuming the leaves and, more rarely, the bark of its hosts. Box tree moth is also sometimes observed on purple-leaved holly (Ilex chinensis Sims, 1819 = Ilex purpurea Hasskarl, 1844 (the name under which it is cited in literature related to Cydalima), dwarf Japanese Euonymus (Euonymus japonicus Thunberg, 1780) and burning bush (Euonymus alatus (Thunberg) Siebold, 1830). However, it seems that to date in Europe, these or related plant species (such as Ilex crenata Thunberg, 1784) are not attacked by C. perspectalis : the rare eggs laid on these species do not give rise to viable larvae (except in the laboratory).

Adults look like the smaller Lomaspilis marginata and caterpillars to those of the large white (Pieris brassicae) © Claude Galand
In Europe, the box tree moth has only been observed on native Buxus : most frequently on (1) common boxwood or evergreen boxwood (Buxus sempervirens Linné, 1753), the most common, widely distributed from Portugal to Germany and Switzerland as well as in the Balkans; more rarely on (2) Balearic boxwood (Buxus balearica Lamarck, 1783) which only lives in the wild in Andalusia, the Balearic Islands and Sardinia. It is also found in abundance on cultivated or directly introduced boxwoods from China such as Buxus bodinieri H. Léveillé, 1913 (Bodinier’s boxwood), Buxus harlandii Hance, 1873 (Harland boxwood), Buxus megistophylla H. Léveillé, 1914, Buxus microphylla Sieb. & Zucca, 1846 (littleleaf boxwood), Buxus rugulosa Hatusima, 1942, and Buxus sinica (Rehder & E.H. Wilson) M. Cheng, 1980 (Korean boxwood).
The moth attacks do not only affect our gardens and parks. Boxwood is also native to Europe and several stands are important elements of many protected Natura 2000 nature sites in Europe. In southern Germany (“Wälder bei Wyhlen”), the only large population of B. sempervirens has now been lost to C. perspectalis mandibles to the extent of more than 95%. Unfortunately, the butterfly has damaged other woods in Italy, France (especially in Alsace and central France) and is also present in wild boxwood sites in Belgium. Further east in Europe, the moth has destroyed many natural forests of Buxus colchica Pojark, 1947 (now considered a synonym for B. sempervirens) typical of the Caucasus mountains, southern Russia and Georgia.
The disappearance of wild boxwood is dramatic in terms of biodiversity but could also be at the root of a major ecological disaster. Indeed, B. sempervirens is known to act on forest succession by influencing differently the establishment and survival of tree species, as in the Pyrenees, where it favours beech over common silver fir (Abies alba). Several studies have also shown that Buxus spp. also grow on steep, friable slopes where they probably play an important role in sediment trapping.
This moth also compromises the aesthetics of the plants it attacks by causing leaf loss, weaving webs and abandoning its droppings. Imagos do not impact boxwood, they feed – like most other butterfly species – on the nectar of different plants, without, it seems, showing a preference for any specific taxon. Adult feeding is very little documented in the scientific literature.
Morphophysiology
Circular and slightly domed, the eggs are initially creamy-white in colour, then quickly turn greenish-yellow with a central orange halo (corresponding to the embryo). Their diameter varies from 0.8 to 1.0 mm. A black dot appears in the egg when the caterpillar’s cephalic capsule begins to form. Hatching occurs about 3 days after egg laying.
At the exit of the egg, the caterpillars measure 1 to 2 mm, they are greenish-yellow with a shiny black head, marked dorsally by a greyish-white “Y”. The 6 thoracic legs are yellowish to brownish (depending on the stage or population). A green stripe flanked by two yellow stripes mark the dorsal side. On the margin of the dorsal segments, a black nipple on the first segments and then two on the following ones, all with white margins and, in the middle, an erect white bristle. Upper half of the lateral surface with a dark green to blackish band width on each segment (except the last ones) a black-green nipple, bearing a long white bristle erect perpendicular to the body.

But on closer inspection, the design is different with the lower half of the side face light green-yellowish © Giuseppe Mazza
Lower half of the side face more or less light green, a long vertical white bristle on each segment as well as on the false legs. Stigmas unpigmented, except on the thorax. Last abdominal segment, basal half of lateral face and ventral face yellow green.
The caterpillars pass through 3 to 7 stages (depending on sunlight and temperature conditions) before reaching their maximum size (between 35 and 40 mm) after 2 to 4 weeks. Finally, it should be noted that these larvae are not stinging.
At maturity, the caterpillars weave a cocoon of white silk among the leaves and twigs of their host plant. The pupa is 25-30 mm long, initially greenish in colour, then darkening and becoming increasingly brownish. Its adult morphological characters become progressively more marked as the chrysalis ages. At the end of pupation, the wings, antennae, eyes, palps and abdominal segments are visible by transparency.

Circular and slightly domed, the eggs are initially creamy-white, then greenish-yellow with a central orange halo corresponding to the embryo. Their diameter varies from 0.8 to 1.0 mm. A black dot appears in the egg when the cephalic capsule of the caterpillar begins to form © Giuseppe Mazza
The chrysalises are always hidden, either within the boxwood or in its immediate vicinity. The temperature threshold for this stage must be higher than 11.5°C.
Note that, as often in butterflies, the sexing of the pupae is not obvious. However, if one compares carefully chrysalis … still it is necessary to have both sexes – the anus is located on the 10th and last abdominal segment in both sexes, and the genital opening of the male on the 9th, while that of the female appears on the 8th, and that of the egg laying on the 9th. The end of the chrysalis is terminated by a clawed organ called “cremaster” which allows the anchoring of the chrysalis at the bottom of the cocoon. The chrysalis stage lasts on average three weeks, except for the last generation chrysalis which will have to overwinter.
The adult moth has white wings bordered with brown and marked by golden to purplish iridescences more or less clear, depending on the angle of incidence of light. The average wingspan varies from 36 mm to a maximum of 44 mm.

Hatching occurs about 3 days after egg laying. At the exit of the egg, the caterpillars measure 1-2 mm. Almost invisible, they quickly hide in the foliage of boxwood © Giuseppe Mazza
The species occurs in three distinct habitats. (1) The most common form, with whitish wings edged with brown. (2) A much less abundant colour variant (one out of 10 individuals in Belgium), with the forewing completely edged with brown (at rest, a brown band seems to divide the wing obliquely). (3) A third morphe (less rare, depending on the region, from 1 to 3 individuals out of 10) that is entirely brown and more or less iridescent, with a white discal spot on the forewing.
All three forms have been observed with certainty in Belgium, the United Kingdom and France. They probably occur in other countries. These different morphs indicate the existence of a certain genetic variability within the established population in Europe and thus indicate extensive adaptive capacities.
It should be noted that different colour forms may appear within the same population. Intermediates also exist between these three well-typed morphs, sometimes complicating the determination of the pest.

Their occurrence is indicated by spots on the foliage and the presence of dung-rich webs with black cephalic capsules resulting from moulting © G. Mazza
Specialists then use examination of the genitalia of male or female moths to confirm their determinations.
Let’s quickly describe the rest of the body in its typical shape. Head is greyish-white to brown, with the back covered with fawn to brown hair, brown-black eyes, light brown to yellowish antennae. The joint between the wings and the thorax is lined with large scales and long white hairs. Thorax and abdomen hairy, whitish in colour (light brown in dark morphs) except for the last brownish abdominal segments. Tarses often white, femurs and tibias white with brown streaks.
Females begin to lay eggs 2 to 3 days after fledging. At first glance the two sexes are identical, but if you look at the abdominal extremities, they differ markedly: a brush of straight black hair finishes the abdomen of the males. Males live half as long as females.

They go through 3 to 7 stages before reaching their maximum size between 35 and 40 mm, after 2 to 4 weeks © Giuseppe Mazza
Ethology-Reproductive Biology
A cycle corresponds to the maximum duration of complete development, from the egg stage to the imago (adult) stage, passing through the various larval stages and ending with the chrysalis.
Box tree moth is polyvoltine, both within its original range and in Europe.
It has at least two development cycles in Central (e.g. Switzerland) and Northern Europe, and often three cycles in warmer regions: the first in early spring (around March-April), the second in summer (around mid-June-July) and the last in early autumn (around September-early October).
Some authors have even recorded a maximum of 5 cycles in warm and humid regions (e.g. China) that overlap over the course of a year. These maximums always correspond to peaks of damage on boxwood. As the cycle of C. perspectalis is directly influenced by the outside temperature and sunlight, it is difficult to describe a typical pattern. We refer the curious to the numerous scientific publications and websites specifying the course of a cycle in the various European and Asian countries.
In Western Europe, under laboratory conditions, the total duration of a cycle is, on average, 45 days under a temperature of 25°C with a day/night alternation of 16 to 8 hours. In nature, the cycle varies between 17 (under optimal conditions of temperature and day length) and 87 days.
Adult breeding females live an average of 12 days (maximum 23 days) compared to an average of 17 days for females with no reproductive activity. Males live about 15 days. The sex ratio is 50% (under laboratory conditions).
Females lay clusters of 5 to 20 eggs on the underside of the wholesome leaves of boxwood, thus limiting competition between larvae and optimizing their development by ensuring their food supply. During their lifetime, they lay (depending on localities and weather conditions) between 190 and 790 eggs (with an average of about 300 eggs).
Egg laying begins very early after the adults emerge. Peak egg-laying is reached as early as the seventh day. The temperature threshold for egg development must be above 10.9°C.
More than 50 % of the eggs are laid between the fourth and tenth day after birth and almost 80 % of the eggs are laid between the fourth and fourteenth day. The fertility study shows that females lay eggs throughout their short lives.

At maturity, the caterpillars begin to weave a cocoon of white silk among the leaves and twigs © Giuseppe Mazza
In the first instar, the caterpillar, due to its small size (1 to 3 mm) is undetectable, remaining on the reverse side of a leaf. It is in the 2nd instar that it moves, starting to weave protective threads of whitish silk. It then becomes increasingly voracious, being able, during the 3rd and 4th instars, to devour both boxwood foliage and bark.
The larvae from a single egg group spread over an area of 20-25 cm in diameter from their egg-laying site.
Optimal temperatures for feeding fluctuate between 15°C and 30°C, depending on the region.
The duration of larval development varies greatly, ranging from 17 to 85 days.
Cycle length is related to the ambient temperature, which should not be lower than 8.4°C, and to sunlight. If the temperature remains mild, all larval instars can still be observed and coexist in autumn.
When the length of daylight falls below about 13.5 hours, the caterpillars (often third instar in Europe) enter diapause (the dormant stage of an insect) and overwinter in a spun cloth, the hibernarium.
These shelters are most often woven between 2 boxwood leaves that are placed side by side and held together. In this state and under this protection, the caterpillars survive temperatures as low as -30 C (in eastern Russia and northern China)!
At the end of winter and early spring, the caterpillars will come out of their diapause and continue their development by consuming the new boxwood leaves, before turning into chrysalis from the end of April, this stage will last between 2 to 4 weeks (sometimes more, if the chrysalis appears in autumn). Depending on the clemency of the winter and depending on the region, adult butterflies will emerge between April and June.
The moth has adapted to multiple climates, situated between two extremes: that of the steppes and that of continental areas. However, several differences have been observed between invasive populations of C. perspectalis and native populations in Asia. The temperature thresholds and degree-days required for the development of eggs, larvae and chrysalises are similar between the European studies, but differ from studies carried out in Japan and elsewhere.
Such dissimilarities can be explained because different geographical biotypes induce distinct developmental responses. In addition, it cannot be excluded that the taxon “Cydalima perspectalis” corresponds to a complex of species and that cryptic species are mixed with the typical form, for example in Japan and continental Asia. Genetic studies will provide an answer to this hypothesis.

At maturity, the caterpillars begin to weave a cocoon of white silk among the leaves and twigs © Giuseppe Mazza
Due to the absence of natural enemies in its European range, populations of C. perspectalis are rapidly becoming very large and dense. They appear to be limited only by food resources and climatic conditions.
This butterfly has a robust and powerful morphology, it moves mainly at night (sometimes, but more rarely, also during the day) and flies over relatively large distances. Its spread is estimated to be a few km or even tens of km per year.
It remains to be determined whether its behavior is sedentary or not. In the areas currently infested, boxwood seems to be quite distant, as it is mainly present in urban areas. These distances therefore seem to slow down the spread of the box tree moth. The possibilities for passive propagation of this pest are, besides the transport of green pruning waste, the trade of boxwood plants by nurseries, garden centers, supermarkets, florists…

The chrysalis instar lasts an average of three weeks. At the end of nymphosis it starts to move © Giuseppe Mazza
Damage detection
Its discreet behaviour makes it easy for the box tree moth to go unnoticed, at least when its population remains low, which is the case when it arrives in new geographical areas.
Hidden under the boxwood leaves, the eggs remain almost undetectable unless the vegetation is examined very carefully. Their small size (± 1 mm) and the small number of eggs laid in clumps make them difficult to find.
The discovery of webs, often greyish-green excrement and even black cephalic capsules resulting from the moulting of the larvae are clear signs of an early infestation of boxwood.
Caterpillars of all stages, even the largest ones (40 mm), hide during the day in boxwood, and only a trained eye will discover them within the dense foliage. They will appear more clearly as defoliation progresses. To search for them, do not hesitate to remove the twigs from dense boxwood.
Discovering the young wintering caterpillars (size ≈ 5 – 8 mm) seems easier, they appear at the apex of the twigs in a protective box – the hibernarium – hidden inside two or three leaves joined together by a few bundles of whitish silk threads. Browning of the foliage is also an early warning symptom of the presence of the moth.
The imagos are not very active during the day and remain hidden within the foliage of boxwood or even another plant. It is not uncommon to observe, in the evening, butterflies resting on walls or lighted windows. This species is, in fact, strongly attracted by artificial lighting, and, more particularly, by certain old bulbs emitting in the ultraviolet spectrum (mercury vapour bulbs, actinic or Wood’s fluorescent tubes).
The chrysalis is always immobile. Within the shrub, it can be easily detected by “palpating” the leaf mass or by gently spreading the leaves apart.
Damage and control
Cydalima perspectalis is the only known pest of boxwood in Europe. This moth is therefore a major threat to nurseries, parks, gardens, but also to boxwood bushes growing spontaneously in forests in various natural ecosystems in Europe.

At the end of nymphosis, dilatation-contraction by absorption of air-pulsion of the haemolymph follows one another. They facilitate the extraction of the imago from its envelope © Giuseppe Mazza
Only caterpillars feed at its expense. Caterpillar browsing causes browning and drying of plant leaves, quickly followed by extensive and rapid defoliation. When the attack remains light, boxwood can produce new leaves, but during outbreaks, boxwood is totally defoliated.
Boxwood usually re-leaves the following season, however, the moth frequently attacks trees during re-leafing, sometimes causing the dieback of some strands.
Adults do not directly impact boxwood, they feed little or not at all (they consume the nectar of many flowers). In acute cases, attacks do not only affect the leaf mass of boxwood, but also the bark and buds (from the fine branching to the branches and stems). It should be noted that, often in urban areas, boxwood dies back in some areas, while other shrubs remain elsewhere almost free.

The adult quickly emerges out of its chrysalis © Giuseppe Mazza
Dense, pruned boxwoods with compact twigs seem to be the most severely affected. Older plants with a natural growth habit and looser branches look spared the most. Perhaps the density of the shrubs protects the caterpillars from their beaked or mandibular predators!?
For the moment this pest has only attacked boxwood in France, but in Asia, the moth also lives at the expense of purple holly (Ilex purpurea), Japanese euonymus (Euonymus japonicus) and Burning Bush (Euonymus alatus).
Boxwood is frequently used for its aesthetic aspect, especially in decorative and private gardens, in topiary art, parks, cemeteries… By feeding on it, the caterpillars of the moth quickly ruin the aesthetic aspect of shrubs and bushes that quickly appear straw-coloured, soiled by greenish droppings and silk threads. Boxwoods are evergreen plants, so the damage caused remains visible for a long time.
In the Caucasus and in many European regions, the moth is gradually eradicating all native populations of Buxus sempervirens, an important component of natural forest ecosystems. This destruction is also likely to have a cascading effect on the species that live exclusively or mainly in this ecosystem.
Several scientific studies have listed a total of 132 fungi, 12 algae, 98 invertebrates and 44 lichens directly related to Buxus species and varieties. Of these, 43 species of fungi, 3 species of algae and 18 species of invertebrates have only been observed on boxwood.
This suggests that all these species would be at risk if Buxus spp. were to disappear from the region.
Basic prevention and control
The box tree moth was imported into Europe with boxwood produced in China, as we saw in the early 2000s. The probability of new introductions from Asia is now lower than in the past because the volume of Buxus traded has recently decreased and several means of control of this pest are, as we will see below, used with varying degrees of success.
All boxwood currently purchased in nurseries or imported is also treated before sale with systemic insecticides that destroy the moths (and other insects)! However, the butterfly continues its ravages in many countries!

Gradually, clinging to the remains of its chrysalis, it swells its wings and dries its cuticle © Giuseppe Mazza
To prevent and limit the spread of C. perspectalis, it is essential to raise public awareness of the risks associated with the movement and purchase of infested boxwood. These evergreen shrubs can be invaded by individuals of all stages of boxwood codling moth. If treatment is not desired, and if few shrubs are infested, the caterpillars can be picked up by hand (they are not stinging) and destroyed immediately (by killing them by fire or freezing). Another way to control the infestation is to shower the boxwood under a pressurized water jet on a clear day! A powerful spray damages “nests” and webs, and dislodges eggs, pupae and caterpillars. It is not 100% effective but can sometimes be enough to save the shrubs.
The gardener can also beat the boxwood with a stick to bring the pests down (remember to put a tarpaulin under the shrub beforehand to collect them). It is also possible to put anti-insect nets on the boxwood to prevent butterflies from laying eggs. The nets – not always very aesthetic – are put down as soon as the first butterflies are detected in the pheromone traps (see below), until the last flight period. This technique is nevertheless risky if you trap caterpillars present in boxwood.

Side view. The adult, clinging to the silk threads that protected it at the caterpillar stage, is now ready to fly away © Giuseppe Mazza
Some studies, which have yet to be evaluated, propose the use of thyme essential oil as a moth repellent, or even the application of a homeopathic treatment based on Psorinum 30 C. These treatments do not kill caterpillars, but globally modify the ecology of boxwood and its environment, making it much less attractive. Professional gardeners, particularly in Haute-Savoie, regularly water boxwood with a fermented extract of nettle and rhubarb leaves or comfrey manure, which are excellent elicitors (phytostimulants) and insect repellents. They complete this preventive treatment with the addition to the substrate, in winter, of a complete fertilizer for trees and shrubs that will strengthen the boxwood. On the other hand, in case of heavy infestations, chemical and/or biological treatments remain essential to get rid of the pest.
Biological control
Populations of the box tree moth are limited within its native range by numerous natural predators and parasitoids. These include species of the genus Aeolothrips (Thysanoptera Aeolothripidae) and the genus Tyndarichus (Hymenoptera Encyrtidae) that consume the eggs (oophagus).

The flight of the adult is not immediate; the moth sometimes remains clinging to the vegetation for many minutes © Giuseppe Mazza
The caterpillars are eaten by at least three Diptera Tachnidae, notably of the genus Exorista and by several Hymenoptera from several families including the Braconidae Chelonus tabonus. These last two taxa, in some regions of China, are thought to parasitize up to 50% of the populations.
The chrysalises have no specific predators, they are nevertheless eaten by insects belonging to several orders, but also by birds and small mammals.
The only parasitoids detected in Europe feeding on C. perspectalis are polyphagous species with little impact on populations. Predation by birds (blackbirds, blue titmouse, starlings, jackdaws…) and insects (chrysope, Asian hornet, wasp…) remains low, probably due to the high levels of toxic alkaloids produced by boxwood and stored by the larvae.
Consequently, these taxa are not useful biological control agents over large areas or during acute attacks.
One species of entomopathogenic nematode (Steinernema carpocapsae Weiser, 1955) has been shown to be effective in the laboratory, but only on caterpillars, pupae and adults were not affected by the worm.
How does this parasite work?
Its larvae enter the caterpillars through the stigmas (respiratory orifices) and rapidly release pathogenic bacteria into the hemolymph. They kill their host within 1 or 2 days. Although already sold to individuals, their use in the field still requires much research. Note that this nematode is not selective, it also kills several other species of insects, whether pests or not, and therefore disrupts the natural balance of the treated area.
Several studies have also focused on the use of entomophagous fungi such as Beauveria bassiana (Bals.-Criv.) Vuill. and a baculovirus (rod-shaped virus, specific to arthropods). Although promising, this research needs to be confirmed in the field, as these organisms are not strict parasites of C. perspectalis either.
Recently, a private firm has put on sale in France kits containing trichogramma containing oophagous larvae that develop inside the egg of the box tree moth. They are barely 0.8 mm long and were chosen for their ability to specifically target boxwood moths. Simple to use, these microhymenopterans would wipe out nearly 90% of the butterfly’s populations, provided they are applied at the beginning of flight and fifteen days later.

Imagos do not impact boxwood, they feed on the nectar of different plants, without, it seems, showing a preference for a specific taxon © Giuseppe Mazza
In 2019, Italian agronomists evaluated the use of Exorista larvarum (Linnaeus, 1758), a fly of the Tachinidae family, fairly common in Europe, as a parasitoid of the box tree moth. Their results in the laboratory are encouraging, the tachinidae can be useful to regulate the pest populations, but these data have yet to be validated in ravaged buxus berries.
Chemical control
Systemic insecticides appear to be very effective against codling moth, but they are harmful to ecosystems, particularly because of their persistence. Moreover, these molecules are not selective and also destroy the natural enemies of the pest, as well as all species that use boxwood as shelter, such as arachnids and other insects. Insecticides that act by contact and ingestion are also very effective and less harmful to butterfly habitats.

A butterfly of the brown form clinging to the vegetation. Different morphs, sometimes intermediate between the three common chromatic forms, testify to the existence of an important genetic variability © Giuseppe Mazza
The most effective are pyrethroids such as deltamethrin and cypermethrin. However, these products are not selective either!
Biopesticides based on Bacillus thuringensis Berliner, 1915 (of the kurstaki serotype) are usually the preferred option (although they kill other moths) on ornamental boxwood because of their limited impact on the environment (the bacterium is present in the soil). They are easy to spray and are not very harmful to humans and other vertebrates. They are ingested by caterpillars that feed on the treated leaves. The toxins produced by the bacillus destroy the cells of the midgut of the caterpillar, which stops feeding and dies within a few days.
The use of recently developed sex pheromone traps captures males of C. perspectalis and drastically limits the reproduction of the species. Pheromones are sexual substances emitted to attract mating partners. They are specific to each insect. Placed in early spring (and then in mid-summer) in the flowerbeds, traps can also be used to estimate peak butterfly abundance and the time of egg-laying. In addition, if the boxwood surface is not too large, an anti-insect net will be placed on the shrub to prevent the females from laying eggs.
In 2019, Hungarian researchers discovered a chemical lure that would attract both males and females of the box tree moth. This discovery suggests interesting prospects for understanding adult populations and controlling this pest, but this research still needs to be refined before the molecule can be commercialized.
Finally, there are two promising research initiatives. The SaveBuxus I (2014-2017) and SaveBuxus II (2018-2020) projects have had and aim to design biocontrol solutions against the codling moth (using Bacillus thurengiensis Kurstaki) by targeting all stages of its development. In specific adults, through trapping and mating disruption. Although the total elimination of this moth now seems illusory, the joint use of different biocontrol solutions could make it possible to maintain its nuisance at an acceptable level.
Finally, it should be noted that grandmothers’ recipes against the box tree moth are false good ideas. There is no point in using vinegar, black soap, baking soda or even bleach. These various products are ineffective against this pest and are sometimes even harmful to boxwood. Not all these means of control are applicable everywhere. A private individual will find it easier to find a solution in his case than a nature reserve manager who is subject to restrictive rules on the use of chemicals and biopesticides.

The disappearance of wild boxwood, devoured by larvae, is dramatic in terms of biodiversity © Giuseppe Mazza
In combination with the biological and chemical solutions presented above, several prophylactic measures can be considered by individuals or managers of natural and non-natural environments to slow down the advance of the box tree moth : reinforce the health of boxwood with phytostimulants and adapted fertilizers (natural or chemical), prune affected areas (then burn the prunings or place them in an active compost), water in hot and dry weather to stimulate the growth of new leaves, place tits boxes near plantations, frequently examine the plants…
Conclusion
C. perspectalis now belongs to the long list of invasive alien species permanently established in Europe. This moth causes significant damage to ornamental and wild boxwood. As it does not pose problems in food production, its economic importance has been underestimated by many field actors.
However, apart from the economic impact it has in the field of ornamental plant production and green space management, its influence on natural areas remains to be clarified. The fight to protect boxwood plantations is proving to be a long-term struggle, the outcome of which is still uncertain.
The lesson to be learnt from this invasion is to be stricter and more reactive in dealing with the routes of import and spread of such parasitic organisms from other continents. BUT … the successors of the box tree moth have already arrived in Europe. We will mention only three of them: (1) the diabolical Halyomorpha halys bug (Stål, 1855), a real scourge for arboriculture, viticulture, market gardening, and other crops, which appeared around 2010, (2) the South American Tomato Leafminer (Tuta absoluta (Meyrick, 1917) which has invaded Europe since 2006 and (3) the Red Palm Weevil (Rhynchophorus ferrugineus Olivier, 1790) which has been devastating palm trees in particular in Spain since 1994, Italy and southern France since 2004 and 2006 respectively, and those of the Principality of Monaco since 2012.
Curiosities
There are no other significant defoliators on Buxus spp. in Europe. However, in some regions, boxwood plants are also severely affected by several moulds (Calonectria pseudonaviculata (= Cylindrocladium buxicola) and C. henricotiae). These parasitic fungi are present from April to October. They form light brown longitudinal streaks in the center of the leaves and much darker ones all around. Eventually, the leaves affected by the disease dry out and the whole twig withers. In case of a major attack, death of the shrub is inevitable.
Synonyms
Phacellura advenalis Lederer, 1863, Glyphodes albifuscalis Hampson, 1899.
→ Per nozioni generali sui Lepidoptera vedere qui.
→ Per apprezzare la biodiversità delle FARFALLE cliccare qui.
→ For general information about Lepidoptera please click here.
→ To appreciate the biodiversity of BUTTERFLIES and find other species, please click here.
